
bone mineral density [71]. Consequently it has been found that fracture
risk in patients is associated with certain geometrical features such as
local thinning of cortical bone [72].
Furthermore, the mechanical competence of cortical bone strongly
depends on its porosity. Cortical bone tissue is composed of osteons
and interstitial bone. The longitudinally oriented Haversian canals and
the perpendicular Volkmann canals perforate the cortical bone matrix.
Towards the endocortical bone surface Haversian canals can unite
and also connect with the intramedullary cavity. The Haversian canals
and the resorption cavities produce a porous bone tissue with pore
diameters ranging from a few up to several hundred micrometers. The
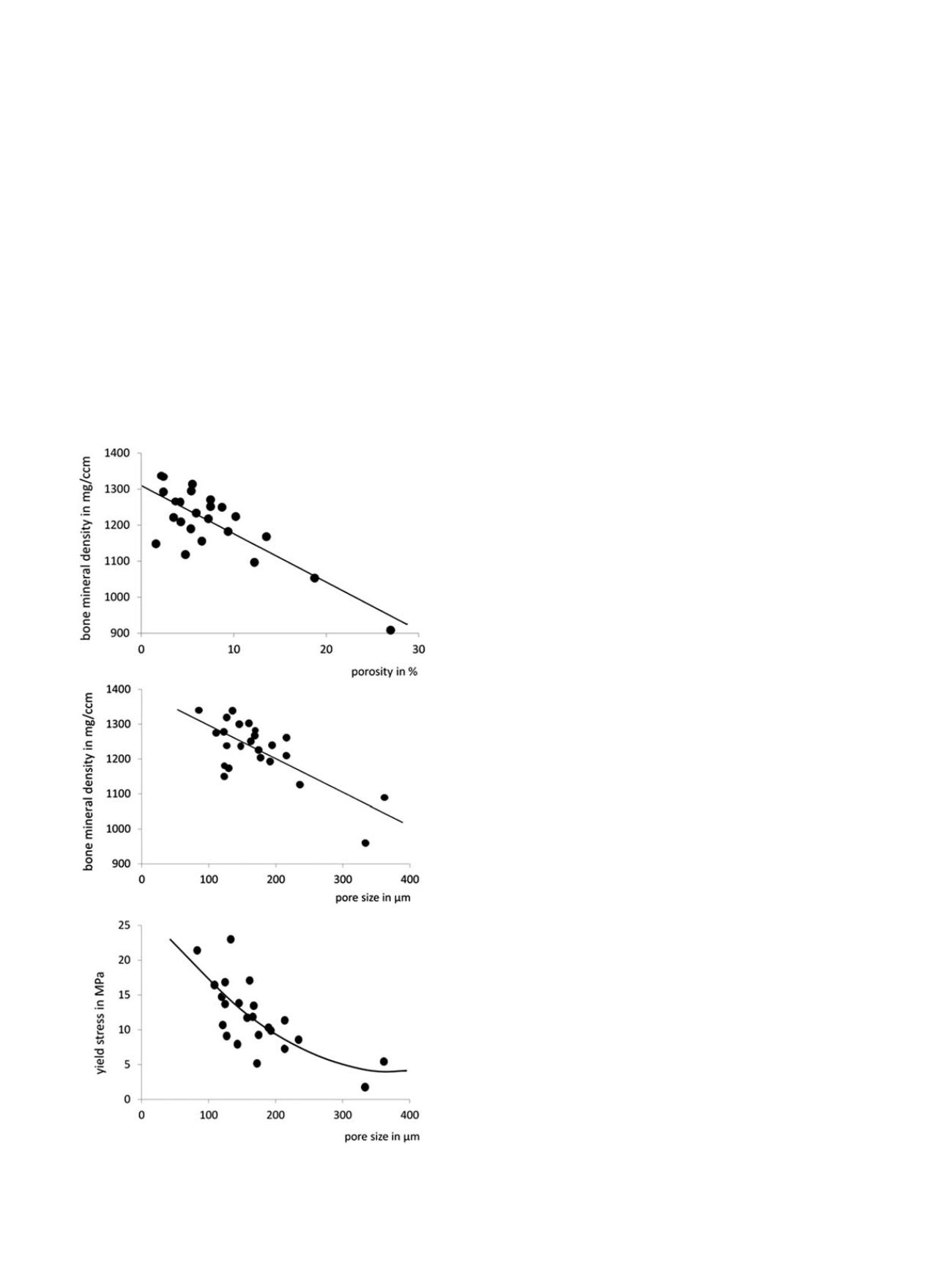
number and size of the pores determine intracortical porosity and bone
mineral density (Figure 7). With increasing pore size the mechanical
properties of cortical bone considerably degrade. Thus porosity
accounts for about 70% of elastic modulus and 55% of yield stress
of cortical bone [73]. Accordingly, fracture toughness also decreases
significantly with increasing porosity possibly by reducing the
available area for the propagation of microcracks [74].
Age-related degradation of mechanical competence of bone
appears to be more pronounced for mechanical properties associated
with failure than for those associated with stiffness. Energy absorption,
fracture toughness and ultimate tensile strain show age-related
decrease of about 5
–
10% per decade, while elastic moduli in tension
or compression degrade by only about 2% per decade [12]. It appears,
therefore, that the relationship between failure properties and stiffness
properties changes with increasing tissue maturity. This makes the
accurate prediction of fracture risk even more difficult. Fracture risk
prediction largely relies on non-invasive image assessment and the
measurement of mineral density. However, while bone mineral density
is closely related to stiffness properties of bones its association with
failure strength or toughness is less pronounced.
Changes in bone
’
s mechanical competence are explained by
functional adaptation of bone structure and age-related deterioration
of intrinsic mechanical properties both being directly related to bone
remodeling. When bone remodeling is suppressed, the ratio of highly
mineralized to new, less mineralized bone tissue is increased resulting
in an increase in the homogeneity of cortical bone tissue. A more
homogenous tissue allows cracks to growmore easily and thus reduces
the toughness of the composite material. Furthermore, remodeling
reduces the regional variability of collagen fiber orientation, leading
to changes in mechanical properties. It has been shown that the
collagen network itself experiences up to 50% loss in its capability to
absorb energy during ageing probably because of an increase in the
percentage of denatured collagen [75]. With increasing age, the degree
of mineralization increases, which is reflected in an increase in mineral
content of cortical bone tissue. As micro-damage in cortical bone
accumulates with increasing age, there is a concomitant progressive
increase in micro-crack density [76]. After the age of 50, micro-cracks
accumulate in cortical bone and this occurs much more quickly in
women than in men.
But not only cortical bone material changes with age, bone
geometry also adapts to a modified mechanical environment. In
essence, both the outer and inner diameter of the cortex increases
while the thickness of the cortex is reduced [77]. In addition, the
porosity of the cortex increases with age and results in a dramatic
increase of the intracortical bone surface. The increase in porosity
results from coalescence of Haversian channels within the cortex and
from fragmentation of the endocortical bone surface. The remaining
cortical remnants have similarity to trabecular bone and can be
described by trabecularization of the endocortical bone (Figure 1). The
porosity in cortical bone increases from about 4% in young healthy
bone to around 12% at age 60 years [14] and up to almost 50% in very
elderly individuals [23]. The increasing surface area of the cortical bone
provides more surface to receive signals for remodeling to be initiated
and thus further accelerates cortical bone loss with age. In fact, most of
the trabecular appearing bone is likely to be trabecularized cortical
bone fragments [78]. While at early ages bone loss dominates at
trabecular sites, with increasing age bone is primarily lost in the cortex
of peripheral bones. Fifty percent of the bone loss occurs at the
endocortical aspect of cortical bone, thinning the cortex and leaving
trabecular like cortical fragments [23].
The adaptive changes of cortical bone tissue with age are largely
site-dependent. In the femoral neck bone loss is lowest in inferior
regions that bear the largest loads during normal gait, whereas regions
at the superior aspect which are less loaded undergo thinning of the
cortex by endocortical absorption. These regions with reduced
thickness however, experience highest stresses during falling and are
more likely to fracture at advanced age. In the femoral shaft, a similar
mechanism has been reported long ago [79]. In the distal forearm, the
age-related adaptation is reflected in endosteal absorption together
with periosteal apposition, increasing the area moment of inertia and
thus preserving bone rigidity and strength [80] to some extent.
Although this adaptive response has been observed in both women
and men, it appears to be more effective in men.
Fig. 7. Cortical bone porosity and mechanical strength
Relationships among bone
mineral density, and pore size in cortical bone and mechanical strength assessed by
yield stress. Data from [4,6]
G. Osterhoff et al. / Injury, Int. J. Care Injured 47S2 (2016) S11
–
S20
S16


















