
The knowledge about these differences between trabecular and
cortical bone and the changes of their relation due to ageing has
multiple potential implications for the understanding and treatment
of osteoporotic fractures. It might be advantageous to apply anti-
resorptive or anabolic medication regimens that aim for modification
of trabecular bone remodelling in younger patients and for modifi-
cation of cortical bone remodelling in the elderly. When a fracture
has occurred, different surgical approaches might be favourable that
either address the
“
trabecular
”
or
“
cortical
”
character of the bone that
is fractured. Bone cement, for instance, which is strong in compres-
sion and weak in shear and tension forces, is an excellent adjunct
tool in the treatment of osteoporotic vertebral or even metaphyseal
“
trabecular fractures
”
[25,26]. In proximal humeral or femoral
“
cortical
fractures,
”
in contrast, a focus on cortical alignment is of more
importance and the use of additional support by cortical grafts might
be beneficial [27,28].
Changes in trabecular bone with osteoporosis and aging
Structural heterogeneity
Even a cursory examination of anatomic sites with high risk of
osteoporotic fracture reveals that bone density and microstructure are
not uniform throughout the trabecular compartment. This regional
heterogeneity in density and microstructure is common knowledge
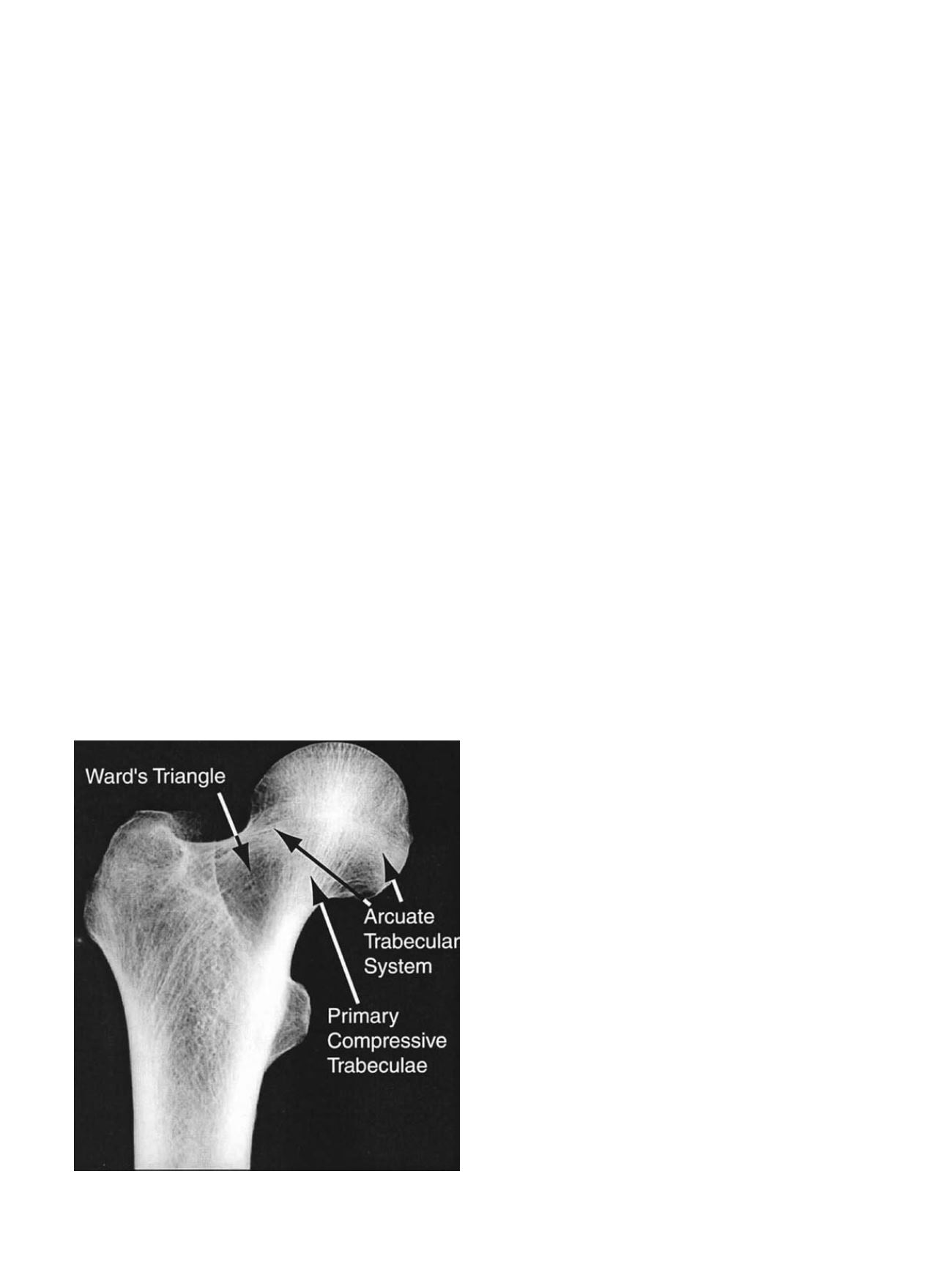
for the proximal femur: Ward
’
s triangle is the region of low density
between the femoral neck and greater trochanter, and the primary
compressive group is the region of high density and strong micro-
structural alignment in the femoral head and neck (Figure 3).
Density and microstructure are also not uniform throughout
the vertebral centrum. Volume fraction and bone mineral density
are highest in the regions of the centrum closest to the endplates and in
the posterio-lateral regions [29
–
34]. Trabecular separation
(Tb.Sp.*)
and degree of anisotropy are highest in the middle and anterior regions
of the centrum [33
–
36]. The relatively low density and high degree
of anisotropy in the anterior region has been suggested as a primary
cause of the high proportion of anterior wedge fractures among
vertebral fractures [37,38]. In addition, the spatial variations in density
and architecture throughout the vertebra change with age [30,35] and
with degeneration of the intervertebral disc [38,39]. Within the
population, bone loss occurs with age at a higher rate on average in
the regions near the endplates than in the central regions
—
resulting
in a more uniform density distribution
—
but the data also show that in
many elderly individuals, the density distribution remains highly non-
uniform [35,37].
The heterogeneity in density and architecture throughout bones
such as the femur and vertebra have been proposed [40
–
43] as a major
reason why the average BMD of the bone explains only
∼
60% of the
variation in whole-bone strength. Biomechanical studies support the
hypothesis that heterogeneity is important for mechanical strength. An
early study using finite element modeling of the femur found that
increases in bone density in a fairly small region (
∼
5 cm
3
) at the
femoral neck could produce a relatively greater increase in bone
strength as compared to a uniform increase throughout the entire bone
[44]. Studies in the vertebra have found that the compressive failure
properties of the vertebra in both static and fatigue loading conditions
were predicted better by measures of density from one or several
sub-regions of the centrum as compared to average density of the
entire centrum [40,41].
However, the literature on the mechanisms by which regional
variations in density and microstructure affect bone strength is mixed.
Studies of excised specimens of trabecular bone have found that failure
in compression initiates in regions of low local volume fraction [45]
and that larger intra-specimen variations in trabecular thickness and
tissue properties are associated with lower apparent elastic moduli
[46,47]. Supporting these findings, Snyder and colleagues have
reported that estimating the weakest cross-section of the vertebral
body provides good predictions of vertebral strength [48,49] and
fracture risk [50]. A study on a small sample of human vertebrae also
reported that increased heterogeneity in volume fraction in the
centrum was associated with decreased compressive strength [51].
In contrast, more recent studies have found that, increased intraver-
tebral heterogeneity in density is associated with
increased
vertebral
strength [52].
Ideally, the measures of heterogeneity that will emerge are those
that have biomechanical underpinnings. For example, increased
intravertebral heterogeneity may confer higher vertebral strength if
this heterogeneity arises from the existence of regions of high density
that are strategically placed in a centrum that is otherwise of low
average density. In other words, larger structural heterogeneity could
be advantageous if the particular spatial distribution of bone density
matches theway that load is distributed throughout the vertebral body.
Prior measurements have shown that in erect spinal postures, less
than half of the total load applied to the vertebral body is distributed
over the anterior half, and that this fraction decreases with age [53].
Vertebral bodies with higher density posteriorly than anteriorly
would be expected to exhibit higher strength under this type of
load distribution, as has been shown [52]. In addition, a prevailing
hypothesis has emerged that degeneration of the intervertebral disc
results in transfer of more of the applied load to the outer regions of the
vertebral body, thus causing resorption in the central and mid-
transverse regions [54]. Vertebrae that have undergone this adaptation
may thus be less likely to fracture [53].
Even considering regional variations in density and microstructure
within small but critical areas of the vertebral body may provide
further insight into the mechanisms of fracture. For example, collapse
of the superior endplate has long been associated with vertebral
fracture, and this collapse initiates in and propagates to regions
overlying trabecular bone of low density and mechanically inferior
microstructure [55] (Figure 4).
In summary, large amounts of heterogeneity in density and
microstructure exist throughout the trabecular compartment of the
bones with high prevalence of osteoporotic fracture. Substantial
Fig. 3. Radiographic frontal view of the proximal femur. Courtesy of Dennis Carter
.
G. Osterhoff et al. / Injury, Int. J. Care Injured 47S2 (2016) S11
–
S20
S13


















